- 22 мая 2024: В п.Новоберезанском забросили аллею Героям СВО
- 9 мая 2022: День Победы 9 мая 2022 года в Кореновске. Фотоотчёт
- 29 апреля 2022: Жители ст.Платнировской остались без медицинской помощи в своей поликлинике...
- 8 апреля 2022: Осуждён телефонный террорист, "заминировавший" Кореновский молочно-консервный...
- 21 марта 2022: В Кореновске сотрудники Росгвардии спасли мужчину из горящего дома
- 2 марта 2022: В Кореновске простились с погибшими в спецоперации на Украине военнослужащими
- 16 января 2022: В Кореновске мужчина повредил машину соседа с помощью серпа и вил
- 21 сентября 2021: В Кореновске 83-летнюю бабушку с переломом перевели в ковидный госпиталь и...
- 23 июля 2021: В центральном парке Кореновска произошла потасовка со стрельбой, трое ранены
- 16 июня 2021: Двух братьев из Кореновского района приговорили к 17 и 15 годам лишения...
Acetone Flashlight - Reaction of Acetone Oxidation , Glowing wire! HD
00:02:10
Подписаться на канал
· 3 подписчика
0 / 0
За ролик пока что никто не голосовал...
Категория ролика: Интересное и занимательное
Теги: познавательное, интересное, занимательное
Hello everyone. Today, we will conduct an interesting experiment, called "acetone flashlight."
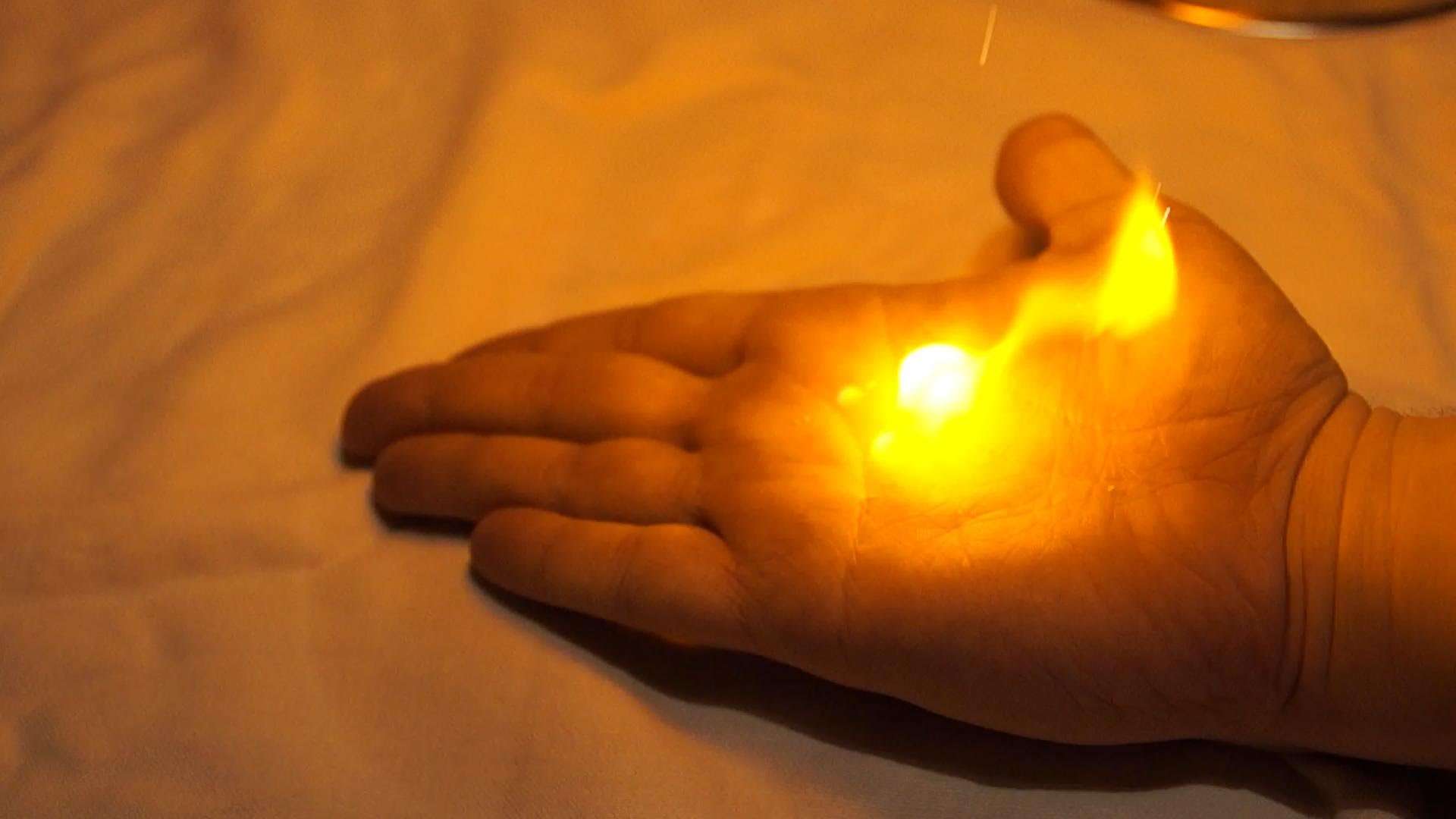
This experiment consists of acetone vapor oxidation at the surface of copper wire, and the wire heats up and glows.
First, you will need to get somewhere a thin copper wire. For a better result, you can use electromotor or solenoid for this, they have a copper coil.
Nip off a small piece of wire and twist it as tightly as possible. The denser are the rolls of barbed wire, the brighter it will glow and the less energy will be lost.
Furthermore, the resulting coil of wire must be burned to get rid of the thin polymer layer.
Once we have prepared the copper wire, pour a little bit of acetone into a beaker.
Next, heat the wire coil with gas burner, and while the wire is going to be hot, immerse it into the cup.
When the wire is lowered into the cup, it begins to warm up and glow.
Acetone vapors are oxidized to acetaldehyde and acetic acid at a surface of the wire. Acetone catalytic oxidation occurs.
If you use a flask instead of the beaker the wire would glow a little brighter.
Due to the fact that the flask has a smaller hole, acetone vapors will come out slower.
Now, let's see how this experiment looks like in the dark.
Even so, substances that are formed in this reaction have sharp and unpleasant smell. Therefore, this experiment should be performed in a well ventilated area or using a fume hood. Facebook:
Patreon:
Music:
This experiment consists of acetone vapor oxidation at the surface of copper wire, and the wire heats up and glows.
First, you will need to get somewhere a thin copper wire. For a better result, you can use electromotor or solenoid for this, they have a copper coil.
Nip off a small piece of wire and twist it as tightly as possible. The denser are the rolls of barbed wire, the brighter it will glow and the less energy will be lost.
Furthermore, the resulting coil of wire must be burned to get rid of the thin polymer layer.
Once we have prepared the copper wire, pour a little bit of acetone into a beaker.
Next, heat the wire coil with gas burner, and while the wire is going to be hot, immerse it into the cup.
When the wire is lowered into the cup, it begins to warm up and glow.
Acetone vapors are oxidized to acetaldehyde and acetic acid at a surface of the wire. Acetone catalytic oxidation occurs.
If you use a flask instead of the beaker the wire would glow a little brighter.
Due to the fact that the flask has a smaller hole, acetone vapors will come out slower.
Now, let's see how this experiment looks like in the dark.
Even so, substances that are formed in this reaction have sharp and unpleasant smell. Therefore, this experiment should be performed in a well ventilated area or using a fume hood. Facebook:
Patreon:
Music:
Комментарии (0)
Нет комментариев. Ваш будет первым!